Saranas’ transcatheter internal bleeding detector scores FDA de novo clearance
Questex LLC | March 08, 2019
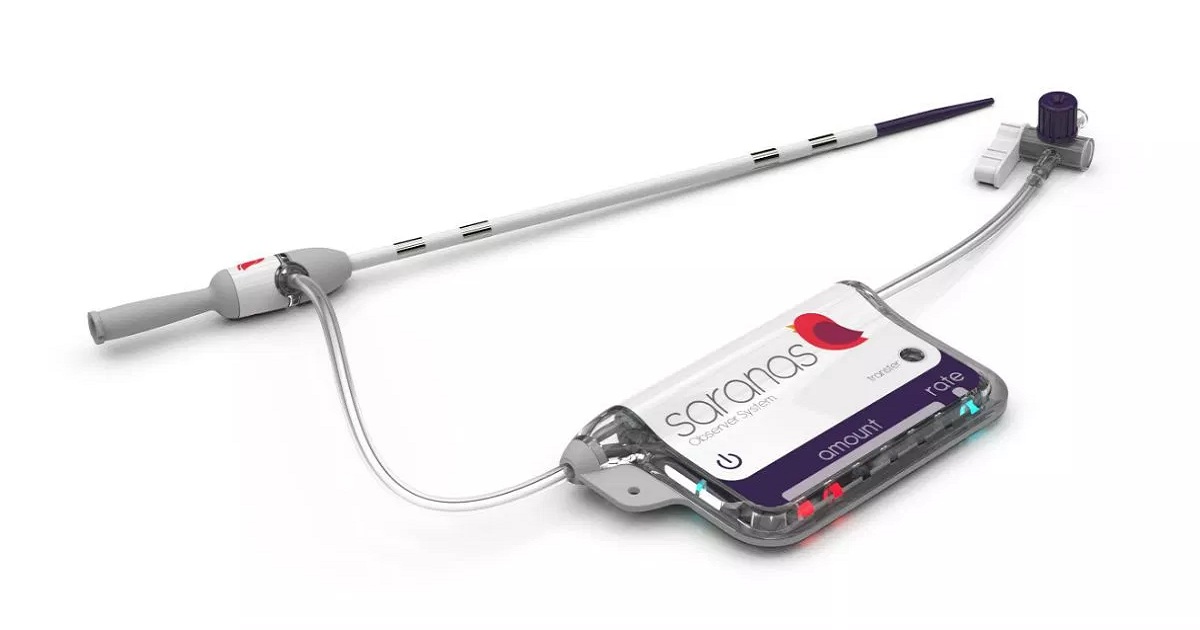
Saranas has received a de novo clearance from the FDA for its Early Bird system, used to detect internal bleeding in real time during endovascular procedures. Invented at the Texas Heart Institute, the device includes a sensor-laden vascular access sheath that measures changes in electrical resistance across the blood vessel. It can alert physicians if the vessel is accidentally torn or injured during transcatheter aortic valve replacements, large-bore hemodynamic support procedures, and other complex interventions where the femoral artery or vein is used. “Peri-procedural bleeding associated with endovascular procedures has a significant impact on patient safety and adds incremental costs to the healthcare system with longer hospital stays and additional interventional treatment,” Saranas’ chief medical officer, Philippe Généreux, M.D., said in a statement. According to the company, a recent study of over 17,000 large-bore interventions found bleeding complications occurred in about 20% of patients.