Roche Buys Ex-U.S. Rights to Sarepta DMD Gene Therapy Candidate SRP-9001 for Up-to-$2.85B+
Genetic Engineering and Biotechnology News | December 23, 2019
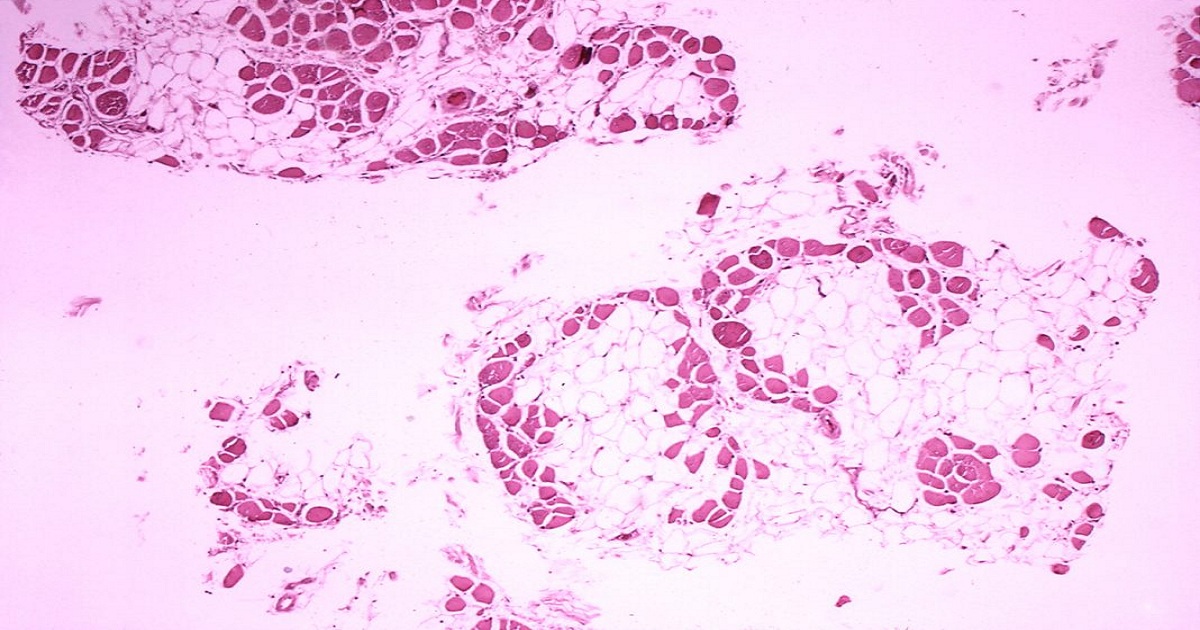
Roche has acquired exclusive commercial rights outside the U.S. to Sarepta Therapeutics’ lead gene therapy pipeline candidate SRP-9001 (AAVrh74.MHCK7.micro-dystrophin) for Duchenne muscular dystrophy (DMD), through a licensing agreement that could generate more than $2.85 billion for Sarepta, the companies said today. SRP-9001, currently in Phase II clinical development, is designed to deliver the micro-dystrophin-encoding gene directly to the muscle tissue for the targeted production of the micro-dystrophin protein. Sarepta is recruiting patients for the two-part Phase II SRP-9001-102 trial (NCT03769116), a 40-patient study designed to assess the safety and efficacy of SRP-9001 in a 48-week randomized, double-blinded, placebo-controlled period (Part 1), and a 96-week, double-blinded extension period (Part 2). The study has an estimated primary completion date of October 10, 2022.