miniPCR bio Announces Launch of the GELATO™ an Integrated DNA Analysis System
miniPCR bio | September 09, 2020
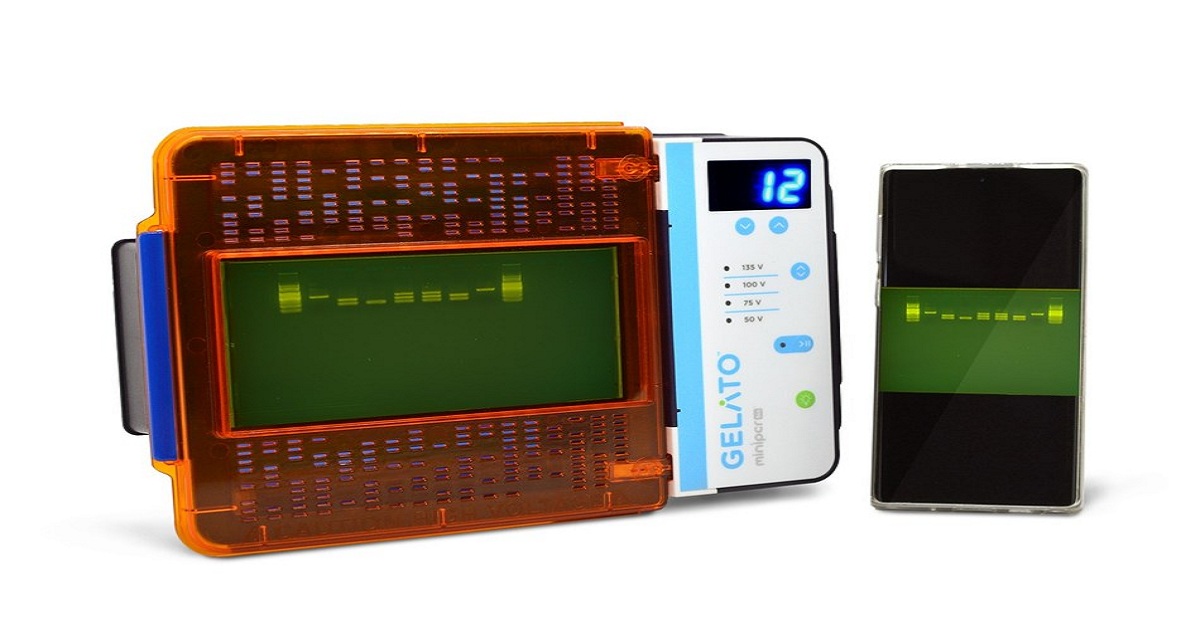
miniPCR bio, a manufacturer of scientific tools for educators and researchers, today announced the launch of the GELATO™, an integrated DNA analysis system. GELATO™ combines gel electrophoresis with transillumination technology, allowing for simultaneous nucleic acid separation and visualization in a single, compact system. As a maker of innovative biotechnology for demanding settings, miniPCR bio has pioneered the development of tools that combine analytical power with the durability and affordability its broad range of users demand. "Our users were asking for a tool that integrates the capabilities of professional lab instruments with the ease of use and compact size of our educational tools," said Dr. Ezequiel Alvarez Saavedra, co-founder of miniPCR bio. "GELATO™ answers that call." Despite a small footprint, GELATO™'s range of casting options allows users to process up to 50 nucleic acid samples at a time. Its variable voltage built-in power supply allows for fast separation of DNA, and its integrated blue-light transilluminator makes it safe for use in all settings. GELATO™ includes a cell-phone compatible documentation hood and features a built-in cutting tray for direct gel excision.