Bioengineers program cells as digital signal processors
tunisiesoir | April 18, 2019
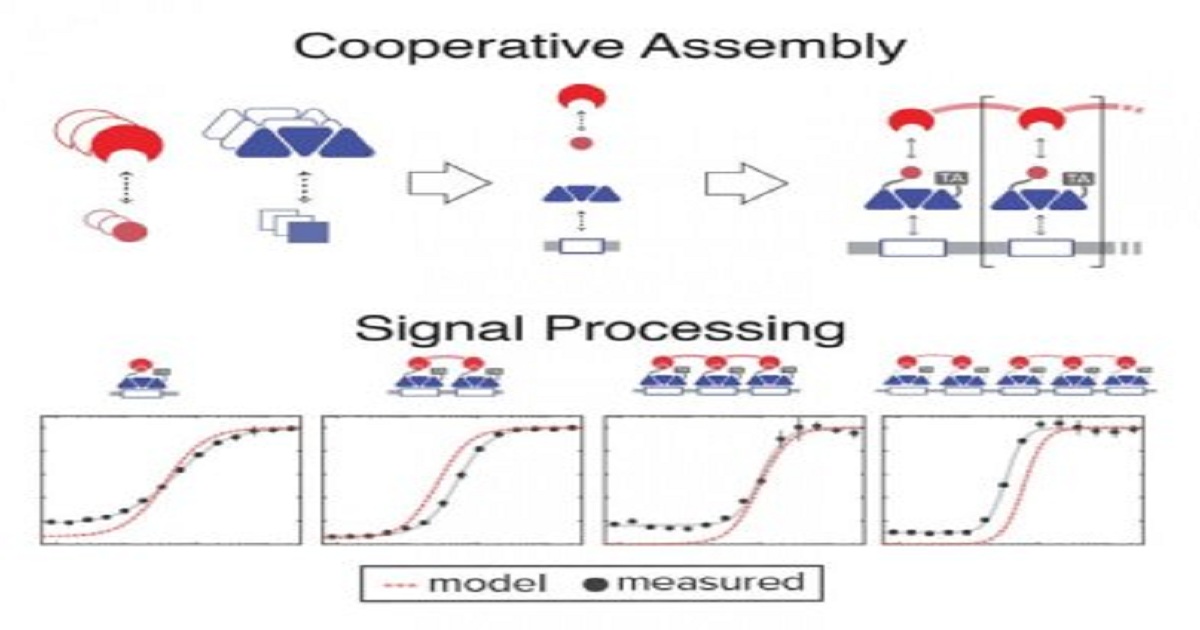
Synthetic biologists have added high-precision analog-to-digital signal processing to the genetic circuitry of living cells. The research, described online today in the journal Science, dramatically expands the chemical, physical and environmental cues engineers can use to prompt programmed responses from engineered organisms. Using a biochemical process called cooperative assembly, Caleb Bashor of Rice University, Ahmad “Mo” Khalil of Boston University (BU) and colleagues from MIT, Harvard, the Broad Institute and Brandeis University engineered genetic circuits that were able to both decode frequency-dependent signals and conduct dynamic signal filtering. “You can think about cooperativity as the same type of signal-processing feature that gives you an analog-to-digital converter, a device that takes something that’s basically linear and turns it into something switchlike,” said Bashor, co-lead author of the study and an assistant professor of bioengineering in Rice’s Brown School of Engineering. Synthetically engineering cooperative assembly allowed the researchers to perform the type of combinatorial signal processing that cells naturally and elegantly do to accomplish intricate tasks, like those in embryonic development and differentiation.