Bioengineering organ-specific tissues with high cellular density and embedded vascular channels
Phys.org | September 17, 2019
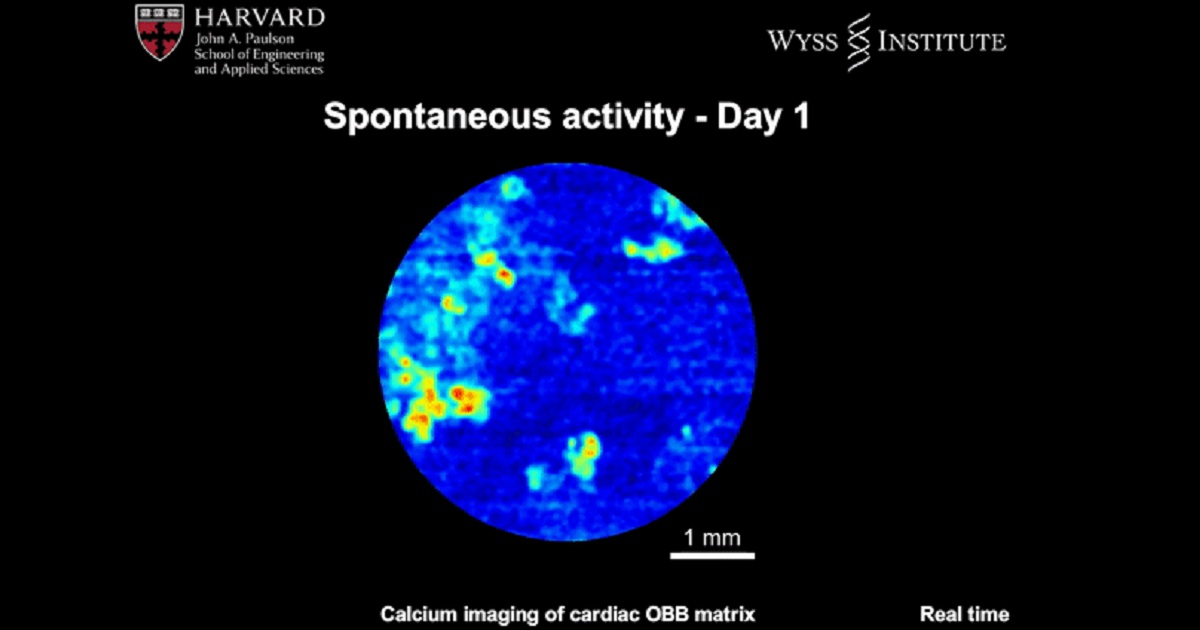
Bioengineers study the development of organ-specific tissues in the lab for therapeutic applications. However, the process is highly challenging, since it requires the fabrication and maintenance of dense cellular constructs composed of approximately 108 cell/mL. Research teams have used organ building blocks (OBBs) composed of patient-specific-induced pluripotent stem cell (iPSC)-derived organoids as a pathway to achieve the requisite cell density, microarchitecture and tissue function. However, OBBs hitherto remain to be assembled into 3-D tissue constructs. In a recent report, Mark A. Skylar-Scott and an interdisciplinary research team at the Wyss Institute for Biologically Inspired Engineering and the John A. Paulson School of Engineering and Applied Sciences at Harvard University, developed a new biomanufacturing method. Instead of 3-D printing constructs to fill in with cells, the scientists assembled thousands of OBBs into living matrices with high cellular density, into which they introduced perfusable vascular channels using 3-D bioprinting. The OBB matrices exhibited the desired self-healing and viscoelastic behavior to convert sacrificial writing into functional tissue (SWIFT). As an example, they engineered a perfusable cardiac tissue to fuse and beat synchronously across a timeframe of seven days. The SWIFT biomanufacturing method allowed the rapid assembly of patient- and organ-specific tissues at therapeutic scales. The research work is now published in Science Advances.